Chimerism - Background Information
The Chimera, according to ancient Greek mythology, was a horrendous fire-breathing monster whose body was composed of parts of a lion, a goat and a snake. Nowadays, the term “chimerism” refers to an organism in which cells derived from different zygotes coexist.
Chimerism can arise in humans through a variety of means, such as inheritance, maternal-fetal stem cell trafficking during gestation, blood vessel sharing in fraternal twin gestation, blood transfusions, bone marrow transplantation, cord blood transplantation, and solid organ transplantation. The presence of two distinct human genomes in a sample can also occur simply through the mixing of human cells from more than one individual, for example, when two cell lines are cross-contaminated, or in forensic tissue samples.
Analysis of chimerism in humans has a significant therapeutic potential in transplantation medicine. It is based on quantitative detection of polymorphic genomic markers such as small insertions/deletions (INDEL), single nucleotide polymorphisms (SNP) or length polymorphisms of short-tandem repeats (STR). Using these markers, a distinct genetic profile is created both for the transplant donor and recipient. Accurate chimerism analysis enables for timely decision-making and provides guidance for effective patient care.
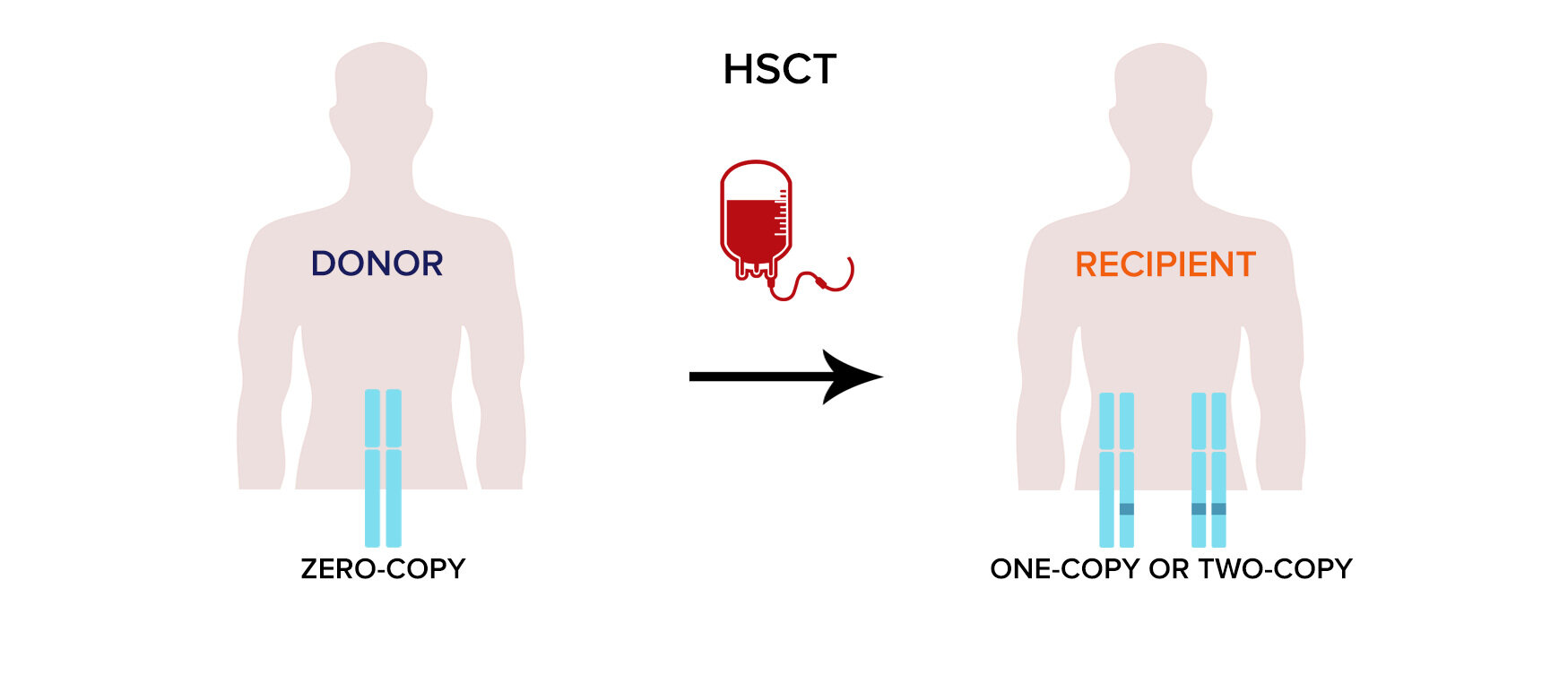
Analysis of chimerism in human medicine. recipient-specific Markers are analyzed following hsct
Chimerism Analysis is Essential After Stem Cell Transfer
Monitoring of chimerism in patients after hematopoietic stem cell transplantation (HSCT) is a crucial diagnostic tool to evaluate engraftment and to asses the risk of disease relapse. Following HSCT, markers that are specific for the recipient are quantitatively detected in a DNA sample from recipient´s peripheral blood and/or bone marrow. Presence of persistent or emerging host cells may indicate imminent disease recurrence.
The QTRACE® Analysis System (CE IVD) for Chimerism Testing
The QTRACE® Analysis System is a CE IVD kit intended for routine monitoring of the levels of recipient-derived white blood cells in peripheral blood and/or bone marrow. It is designed to detect distinct small insertion/deletion (INDEL) markers in the genome by the highly sensitive qPCR methodology. The QTRACE® Analysis System meets the needs of any research application that requires highly sensitive detection and quantification of the genome of one individual in the background of another individual or individuals.
The QTRACE® Analysis System uses qPCR technology and consists of DNA genotyping plates, 46 individual quantification assays, one reference assay, and QTRACE® Software. The QTRACE® INDEL Assays are able to differentiate, and then quantify, the contributors to a human-mixed DNA sample.